Abstract
Background: Treatment decision-making for elderly DLBCL patients (pts) is challenging. Advancing age is associated with an increase in the incidence of comorbidity and vulnerability to treatment-related toxicities (Thieblemont & Coiffier. J Clin Oncol 2007). Mosunetuzumab (M) is a CD20xCD3 T-cell engaging bispecific monoclonal antibody (Ab) that redirects T cells to eliminate malignant B cells. The Phase Ib/II GO40516 study (NCT03671018) is evaluating M in combination with the anti-CD79b antibody-drug conjugate polatuzumab vedotin (Pola; M-Pola) in pts with B-cell non-Hodgkin lymphoma (B-NHL). In the ongoing Phase II part of the study, M-Pola showed an acceptable safety profile with promising activity in pts with R/R B-NHL (Budde et al. ASH 2021). Here, we present a subgroup analysis of the efficacy and safety of M-Pola in pts aged <65 and ≥65 years with R/R aggressive B-NHL from the Phase Ib dose-escalation and Phase II dose-expansion cohorts.
Methods: All pts in this analysis had DLBCL (including transformed follicular lymphoma and follicular lymphoma grade [Gr] IIIB), an Eastern Cooperative Oncology Group performance status (ECOG PS) 0-2, and ≥1 prior line of therapy, including an anti-CD20 Ab. Treatment cycles were 21 days. Pola (1.8mg/kg intravenous [IV] infusion) was given on Day (D)1 of Cycle (C)1-6. M (IV infusion) was given with step-up dosing during C1 to mitigate cytokine release syndrome (CRS), and the recommended Phase II dose was used in the Phase II dose-expansion cohort (1mg on C1D1, 2mg on C1D8, 60mg on C1D15 and C2D1, and 30mg on D1 of C3-8). Pts with stable disease or a partial response at the end of C8 could continue M for a total of 17 cycles, while pts with a complete response (CR) discontinued M after C8. In the Phase II expansion cohort, mandatory hospitalization was not required for M administration. Response was assessed by investigators using Lugano 2014 criteria (Cheson et al. J Clin Oncol 2014). CRS events were reported using American Society for Transplantation and Cellular Therapy criteria (Lee et al. Biol Blood Marrow Transplant 2019).
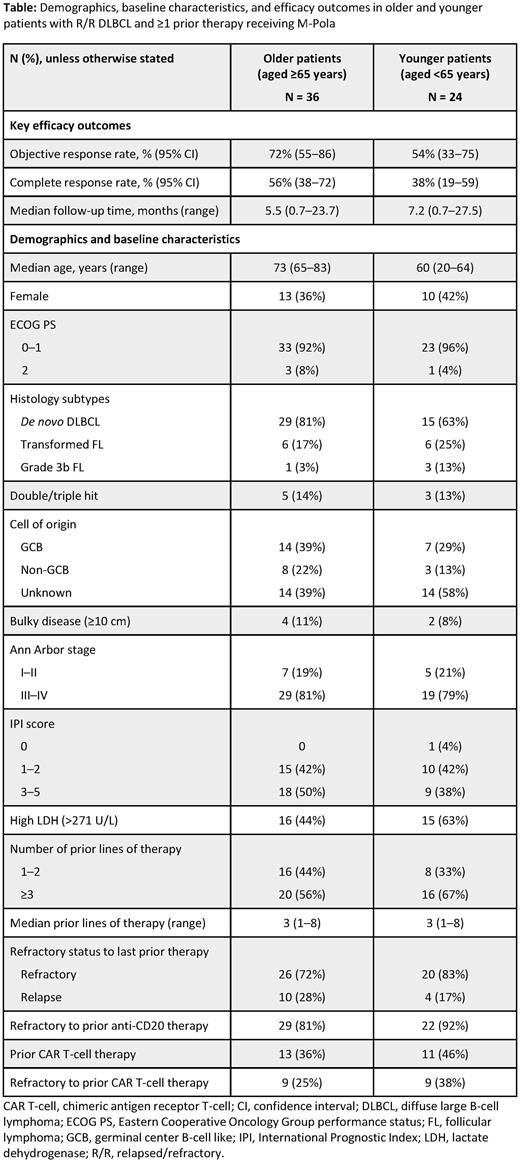
Results: As of March 15, 2021, with a median follow-up of 5.3 months (range: 0.7-23.7 months), 60 pts with R/R DLBCL had received M-Pola: 24 (40%) were aged <65 years and 36 (60%) were aged ≥65 years. At study entry, for pts aged ≥65 years, the median age was 73 years (range: 65-83), 53% had ECOG PS 1 and 8% had PS 2, 50% had International Prognostic Index 3-5, 81% had Ann Arbor stage III/IV disease, and 81% and 72% were refractory to a prior anti-CD20 Ab and to last prior therapy, respectively. There were more pts <65 years than pts ≥65 years with high lactate dehydrogenase (LDH; 63% vs 44%, respectively), with ≥3 prior lines of therapy (67% vs 56%), and refractory to CAR T-cell therapy (38% vs 25%; Table).
Compared with younger pts, those aged ≥65 years had a numerically higher ORR (72% [95% CI: 55-86] vs 54% [95% CI: 33-75]) and CR rate (56% [95% CI: 38-72] vs 38% [95% CI: 19-59]).
The rate of Gr 3-4 adverse events (AEs) was lower in pts ≥65 years than in those <65 years (39% vs 58%, respectively), with fewer neutropenia events occurring (14% vs 33%); the rate of serious AEs of any Gr was similar (39% vs 33%). Five pts ≥65 years discontinued M and/or Pola due to treatment-related AEs compared with one pt <65 years. The rate of CRS was comparable in pts aged ≥65 and <65 years (17% vs 21%), all CRS events were low-Gr (≥65 vs <65 years: Gr 1, 14% vs 21%; Gr 2, 3% vs 0%), and all CRS events resolved. Rates of serious AEs of infection were comparable in pts aged ≥65 and <65 years (11% vs 17%, respectively). Rates of peripheral neuropathy were 42% in pts aged ≥65 years vs 21% in pts aged <65 years, with most events Gr1-2 (≥65 vs <65 years: 1 vs 2 Gr 3 events). After the first M-Pola dose, pharmacodynamic changes in interferon-α and T-cell activation in peripheral blood were similar across age groups.
Conclusions: M-Pola is effective and has a manageable safety profile in pts with heavily pretreated DLBCL, including older pts who may have more comorbidities and fewer treatment options, such as those who are poor candidates for CAR T-cell therapy. Numerically higher ORR and CR rates were seen in pts aged ≥65 years, although longer follow-up is required. Comparable rates of CRS and serious AEs were observed across age groups. Enrollment in the Phase II study with no mandatory hospitalization is ongoing.
Disclosures
Chavez:ADC Therapeutics: Research Funding; TG Therapeutics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Epyzime: Honoraria; Astrazeneca: Research Funding; BeiGene: Honoraria; Merck: Research Funding; Abbvie: Consultancy; Kite, a Gilead Company: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen: Research Funding; AdiCet: Consultancy; GenMab: Consultancy. Ghosh:TG Therapeutics, Genentech/Roche, Bristol Myers Squibb, Gilead, Morphosys, AbbVie: Research Funding; Gilead, AstraZeneca, Bristol Myers Squibb, Phamacyclics, Janssen, Epizyme: Speakers Bureau; Seagen, TG Therapeutics, AstraZeneca, Phamacyclics, Janssen, Bristol Myers Squibb, Gilead Sciences, Beigene, Incyte, Karyopharm, Roche/Genentech, Novartis, Loxo Oncology, Genmab, Adaptive Biotech, ADC Therapeutics: Consultancy. Kamdar:AbbVie, AstraZeneca, Celgene/ Bristol-Myers Squibb, Adaptive Biotechnologies, ADC therapeutics, Beigene, Genentech, Impact bio: Consultancy; TG Therapeutics, Genentech, Novartis: Research Funding; SeaGen: Speakers Bureau; Celgene, Genentech: Other: DMC. Lossos:NCI: Research Funding; Adaptive: Honoraria; LRF: Membership on an entity's Board of Directors or advisory committees. Sabry:AstraZeneca, BMS, Abbvie, Novartis: Honoraria; MD Management, Edward Jones financial services: Divested equity in a private or publicly-traded company in the past 24 months. Dorritie:Kite, BMS, Janssen, Genmab, Hoffman-LaRoche: Research Funding. Huw:Genentech, Inc.: Current Employment. Pham:F. Hoffmann La Roche, Ltd.: Current Employment. Jia:F. Hoffmann La Roche, Ltd.: Current Employment, Current equity holder in publicly-traded company. Wu:F. Hoffmann La Roche, Ltd.: Current Employment; Amgen: Ended employment in the past 24 months. To:Genentech, Inc.: Current Employment, Current equity holder in publicly-traded company. Wei:Genentech, Inc.: Current Employment; F. Hoffmann La Roche, Ltd.: Current equity holder in private company, Current holder of stock options in a privately-held company, Patents & Royalties. Assouline:Novartis: Research Funding; Genentech/Roche, Astra Zeneca, Novartis, BMS, Jazz, Gilead, Amgen, Beigene, Abbvie, Paladin: Consultancy, Honoraria.
OffLabel Disclosure:
Mosunetuzumab is a CD20xCD3 bispecific antibody that redirects T cells to engage and eliminate malignant B cells. Mosunetuzumab is an investigational agent in the United States.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal